For this reason, the international association for the study of lung cancer (iaslc) convened a multidisciplinary panel of thoracic oncology experts with interest and expertise in liquid biopsy and molecular pathology to evaluate currently available evidence with the aim of producing a set of recommendations for the use of liquid biopsy for molecular analysis in. In the past, some lung cancer patients could not take advantage of the latest discoveries in personalized medicine because there was not enough tissue to do biomarker testing.
Rapid developments have led to multiple platforms for isolating circulating tumor cells (ctcs) and detecting circulating tumor dna (ctdna);
Liquid biopsy lung cancer. A liquid biopsy is a simple blood test that is increasingly being used to help detect and treat lung cancer. • at the moment, liquid biopsies are recommended when a tissue biopsy is difficult, such as in the case of lung cancer, or when the original site of the disease is unknown. For this reason, the international association for the study of lung cancer (iaslc) convened a multidisciplinary panel of thoracic oncology experts with interest and expertise in liquid biopsy and molecular pathology to evaluate currently available evidence with the aim of producing a set of recommendations for the use of liquid biopsy for molecular analysis in.
However, the estimated clonal mutant allele frequency (maf) in ctdna of potentially curable lung cancers,. A new companion to lung cancer treatment. Ultimately, notwithstanding the enthusiasm encompassing liquid.
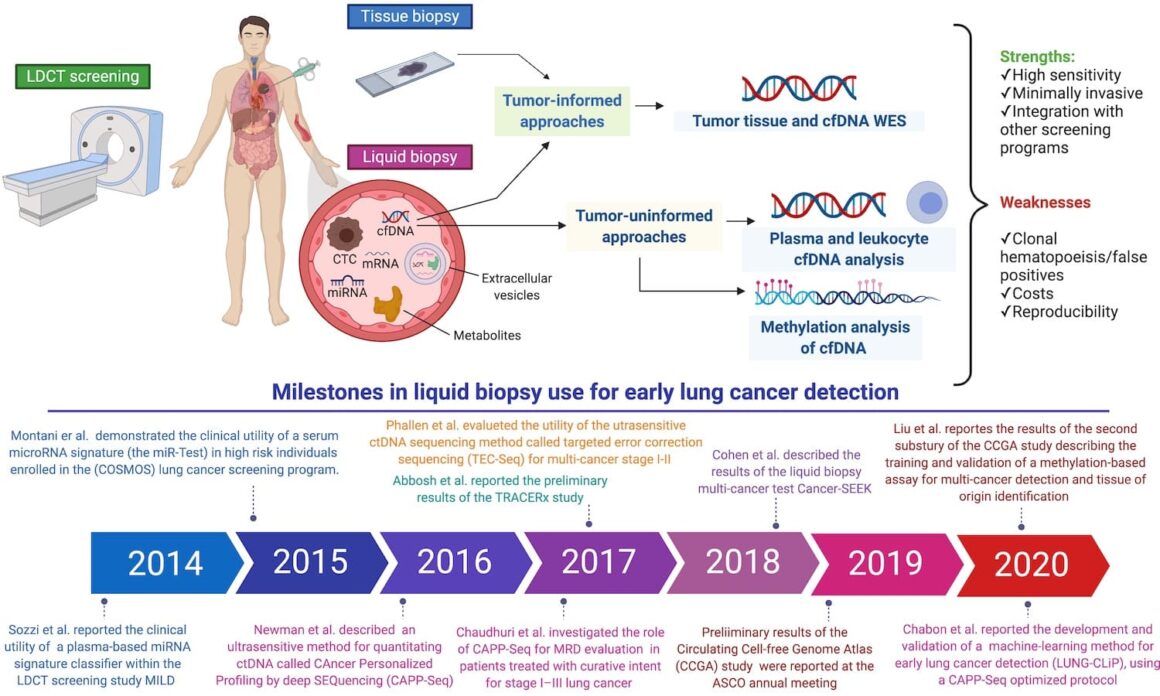
Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages nicolas guibert1,2,3, anne pradines 2,4, gilles favre2,3,4 and julien mazieres1,2,3 affiliations: This review will focus on new liquid biopsy technologies and how they may assist in lung cancer detection, diagnosis, and treatment. Liquid biopsy has not been validated for lung cancer diagnosis, and its lower sensitivity could lead to significant diagnostic delay if liquid biopsy were to be used in lieu of tissue biopsy.
Rapid developments have led to multiple platforms for isolating circulating tumor cells (ctcs) and detecting circulating tumor dna (ctdna); Multiple liquid biopsy molecular methods are presently being examined to determine their efficacy as surrogates to the tumor tissue biopsy. A new and exciting way to identify biomarkers, liquid biopsies are a type of blood tests.
In such cases, dr pathmanathan said, liquid biopsy has proven to be a viable alternative for many of his lung cancer patients. This concept is particularly relevant in lung cancer as the tumour is often difficult to reach and may need an invasive and potentially harmful procedure. With fda approval of the first liquid biopsy in 2016 and advances in research.
Liquid biopsies, particularly ctcs and cfdna, will guide treatment decision, improve the outcome for lung cancer patients and allow early diagnosis of tumors that are not yet visible on imaging. Several studies have evaluated the role of ctdna for early lung cancer detection. Liquid biopsy and lung cancer.
In the past, some lung cancer patients could not take advantage of the latest discoveries in personalized medicine because there was not enough tissue to do biomarker testing. Biomarker identification is most commonly based on surgical sample collection. Liquid biopsies also have a potential role in identifying patients at risk of relapse post treatment and as a component of future lung cancer screening protocols.
2cancer research centre of toulouse (crct), inserm, university. Ctcs and ctdna are the two materials most commonly analyzed in lung cancer “liquid biopsies”. 9 although not recommended as a replacement for a diagnostic tissue biopsy, liquid biopsy is recommended.
Liquid biopsies have a powerful role in helping patients get to the right treatment. Scientists can use liquid biopsies to determine whether lung cancer has started to spread round the body. Moreover, the multitude of anticancer drugs and their sequential use underline the importance of conducting.
This assay demonstrated 98% concordance with tissue in clinical validation. This meeting will offer both traditional sessions and breakout workshops. Liquid biopsy has not been validated for lung cancer diagnosis, and its lower sensitivity could lead to significant diagnostic delay if liquid biopsy were to be used in lieu of tissue biopsy.
Ultimately, notwithstanding the enthusiasm encompassing liquid biopsy, its clinical utility remains unproven. 1thoracic oncology dept, hôpital larrey, university hospital of toulouse, toulouse, france. Currently, liquid biopsy, in particular ctdna, can indicate better tumor heterogeneity at a greater accuracy compared with tumor biopsy, since it facilitates a convenient and dynamic analysis (42,43).
Integration of these and other promising liquid biopsy tests, for example, based on microrna 9 or metabolomics 10 profiles, with current screening programmes might considerably improve lung cancer. For the longest time, the surest way to confirm a cancer diagnosis entailed cutting pieces of tissue out from the affected area for examination, otherwise known as a tissue biopsy. The question is how liquid biopsies can be utilized for immunotherapy.
Biomarkers associated with liquid biopsy for lung cancer immunotherapy. The purpose of the test is to identify patients who may be candidates for treatment with erlotinib (tarceva®) ® and osimeritinib (tagrisso®) ® — targeted therapies that attack cancer cells with egfr. Liquid biopsy is recommended in the new college of american pathologists (cap)/international association for the study of lung cancer (iaslc)/association for molecular pathology (amp) guideline for molecular testing of patients with nsclc.
In 2016, fda approved a liquid biopsy test, called the cobas® egfr mutation test for the detection of egfr gene mutations in ctdna of patients with lung cancer.