About 30% of patients with muc will respond to icis immunotherapy. The most common cancers in this cohort were bladder, head and neck, lung, and kidney cancer, as well as melanoma.
A new study, published in nature medicine, identifies the histone methyltransferase g9a (also known as ehmt2) as a new.
Checkpoint inhibitors bladder cancer. We herein report the trials investigating these checkpoint inhibitors (pembrolizumab, nivolumab, atezolizumab, durvalumab and avelumab) in this particular setting. Checkpoint inhibitors are agents that will regulate the immune system. The food and drug administration (fda) in recent weeks has approved four immunotherapy drugs for bladder cancer, bringing the total number of approved immunotherapies for this disease to five.
Therefore, we aimed to elucidate the association between icis in bladder cancer and ataxia telangiectasia mutated (atm),. It certainly makes sense to look at checkpoint inhibitors in this particular area. Clinical studies to explore ici applicability in the neoadjuvant setting are currently ongoing, with at least two of them that have reported their results.
The treatment of advanced metastatic urothelial carcinoma has recently evolved with the approval of five checkpoint inhibitors. This morning, roche announced it was voluntarily withdrawing the u.s. Immune checkpoint inhibitors (icis) have shown promising results in bladder cancer (bc).
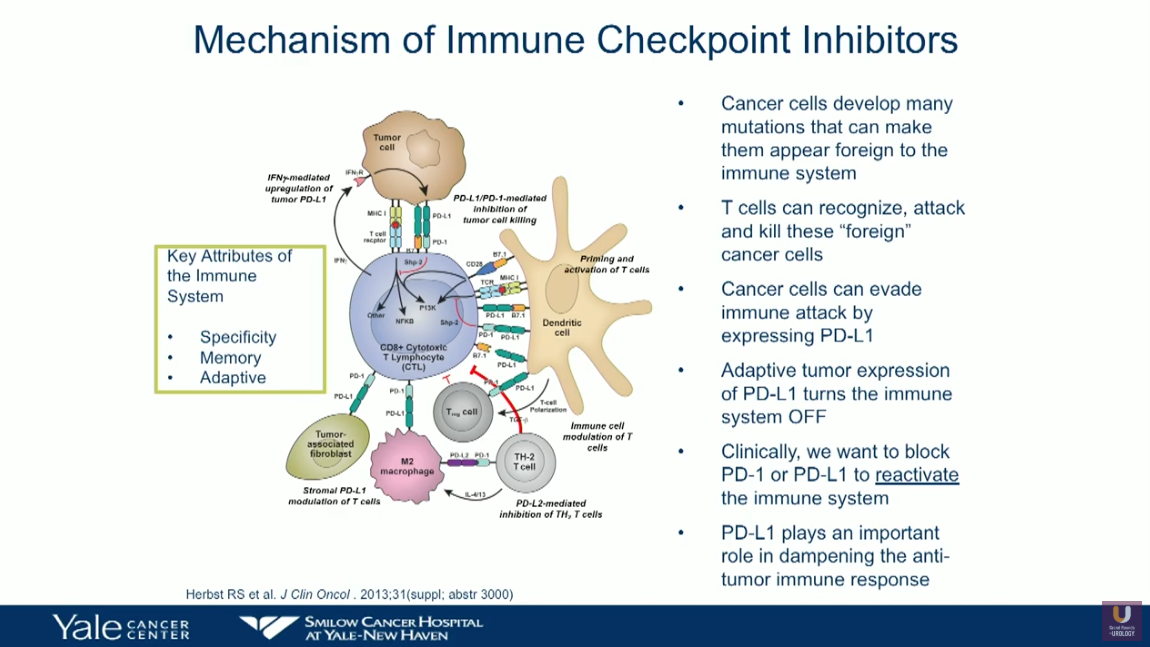
The results that i’m describing were originally published in nature about a year ago and i’m going to show you some update. Here, you can see the pathway of immune regulation. Immune checkpoint inhibitors are approved to treat some people with a variety of cancer types, including:
Most patients (79.7%) were white, and 56.1% were younger than 65 years of age. This is due to immunological cell death. Atezolizumab and pembrolizumab can be used in people who can�t get the chemo drug cisplatin (due to things like hearing loss, kidney failure, or.
Bladder cancer, chemical carcinogens we also know that smoking is a risk factor and it has a high mutation rate. Immunotherapy with immune checkpoint inhibition (ici) has provided durable treatment responses in advanced, metastatic, bladder cancer patients. However, only some patients respond to icis.
A new study, published in nature medicine, identifies the histone methyltransferase g9a (also known as ehmt2) as a new. Dna repair defects (ddr) play an important role in the therapeutic response of bladder cancer. The first trials using checkpoint inhibitors before surgery, when the cancer is still confined to the pelvis, without signs of metastasis, have reported promising results.
Bladder cancer (bc) is the most common malignancy of the genitourinary tract, with high morbidity and mortality rates. Since 2016, five immune checkpoint inhibitors (icis) have b. Revolutionary advances for treating urothelial carcinoma.
About 30% of patients with muc will respond to icis immunotherapy. New bladder preserving strategies are needed for muscle invasive bladder cancer (mibc). Immune checkpoint inhibitors for bladder cancer:
Pembrolizumab and atezolizumab are both immune checkpoint inhibitors, and both are approved by fda to treat patients with metastatic bladder cancer that has continued to progress on standard treatments such as combinations of chemotherapy drugs. The pathway starts when cancer cells release antigens into the lymph node and into circulation. We conducted a systematic review according to the prisma statement for data published on the clinical efficacy of checkpoint inhibitors in metastatic bladder cancer.
The treatment of advanced metastatic urothelial carcinoma has recently evolved with the approval of five checkpoint inhibitors. Combining epigenetic and immune checkpoint inhibitors in bladder cancer. Until recently, the treatment of locally advanced or metastatic urothelial bc was based on the use of chemotherapy alone.
Straightforward submission service, including a free language check on your manuscript. Two weeks after astrazeneca withdrew the use of imfinzi as a treatment for bladder cancer in the united states, swiss pharma giant roche is following suit with its checkpoint inhibitor tecentriq (atezolizumab). Immune checkpoint inhibitors (ici) have demonstrated a higher benefit in heavy cd8 immune cell infiltrated tumors and in tumors with high tumor mutational.
Known as checkpoint inhibitors, all four drugs work by “releasing the brakes” on the immune system and allowing immune cells to attack tumors. Renal cell cancer (a type of kidney cancer) skin cancer; Any of these checkpoint inhibitors can be used in people with advanced bladder cancer that starts growing again after chemotherapy.
Atezolizumab was administered in two cycles before surgery in a single arm, phase 2 neoadjuvant trial known as abacus, with a ratio. The most common cancers in this cohort were bladder, head and neck, lung, and kidney cancer, as well as melanoma. About 30% of patients with muc will respond to icis immunotherapy.